51 questions and answers, a thorough TORCH screening during pregnancy!
TORCH screening is mainly to check whether the pregnant female mother is infected with Toxoplasma gondii, rubella, cytomegalovirus, herpes simplex virus and other pathogens.
First, the misunderstanding of TORCH screening
01、Can TORCH screening diagnose birth defects?
I can’t.TORCH screening is the diagnosis of pregnant women’s infection, which provides attention to whether the fetus has infection and developmental defects. Screening is to screen out the high-risk individuals (infected persons) of a certain disease (virus) from the crowd (pregnant women), diagnose the latter (diagnosis of fetal infection), and intervene the patients (fetus) or disease (virus) carriers (pregnant women) to achieve the purpose of prevention and treatment.
02、What are the common characteristics of TORCH infection?
1, mother-to-child transmission, T1 fetal risk, T3 neonatal risk.
2. Pregnant women are asymptomatic or have mild symptoms.
3, the virus can cause intrauterine infection through the placenta, which can cause premature delivery, abortion, stillbirth or teratoma.
4. The virus infects the newborn through the birth canal or breast milk, causing multiple system and organ damage and mental retardation.
5, pregnant women are infected, the fetus is not necessarily infected, and fetal infection does not necessarily cause birth defects.
03、TORCH infection difference?
After infection, the serological changes of Toxoplasma gondii, rubella virus, cytomegalovirus and herpes simplex virus are different (Figure 2), and the antibody changes are also different. It is necessary to quantitatively detect the degree of antibody changes to make a correct judgment.

04. What are the clinical significance of TORCH detection indicators?
1. Direct indicators (virus antigen, virus DNA, virus RNA virus culture) detect the virus itself, which is related to the characteristics of virus replication and latent position, and is suitable for diagnosis.
2. Indirect indicators (IgG, IgM) detect the immune response of the body after the virus stimulates the body, which is related to the individual’s immune function and is suitable for screening and immune state evaluation.
05. How many kinds of infections are there during pregnancy?
Infection during pregnancy can be divided into primary infection, past infection, recurrent infection and reinfection, and their concepts cannot be confused.
1. Initial infection
Primaryinfection is also called primary infection: pregnant women’s serum is positive for virus-specific antibody IgG for the first time, while previous serological tests are negative, which is called primary infection. Only when screening has been done before and the results are negative and archived (or the serum samples of pregnant women are preserved) can the first infection be judged.
In the case of IgG positive, the affinity test of IgG antibody is helpful to distinguish whether it is acute primary infection, previous infection or recurrent infection, and to estimate the time of primary infection, that is, if the test result is high affinity, it can be concluded that the time of primary infection is 3 months ago.
2. Past infection
Past infection: once infected with the virus, the body produced antibodies or the virus was dormant.
3. Recurrent infection
Recurrent infection/secondary infection: The intermittent excretion of virus in the presence of host immune function is the reactivation of endogenous virus in latent state.
4. Reinfection
Reinfections: Individuals who have been immunized are exposed to a new exogenous virus and reinfected. At present, recurrent infection and reinfection cannot be distinguished by serological methods, but only by virus isolation and molecular biology methods.
5. Congenital infection
Congenital infections: the result of the transmission of the virus through the placenta. The mother’s primary or recurrent infection can spread the virus to the fetus, resulting in congenital infection of the fetus.
06. Why should IgG and IgM be screened at the same time?
Screening experiment of IgG and IgM should be done at the same time, and IgM alone often gives wrong results. IgM positive can not fully prove the recent infection. IgM can exist for several years after infection in some people, and IgM positive alone can not be diagnosed. For example, IgM infected with non-acute rubella can be divided into the following two situations:
1. IgM is really positive
This is because some people have been expressing IgM for many years after infection, and the IgM level often keeps a low and stable level, often accompanied by IgG positive and also keeps a stable level. At this time, the IgM results detected by the kit are correct, but it does not mean that the individual is suffering from acute infection. At this time, if IgM is detected alone, it will lead to misjudgment.
2. IgM false positive
This is mainly due to the influence of factors such as rheumatoid factor (RF), cross-reaction or polyclonal stimulation, which leads to the error of the test results of the kit. This is due to the inherent limitations of immunological testing methods and cannot be completely avoided.
The IgM positive results of non-acute infection caused by the above two conditions will bring confusion to clinical diagnosis, because these individuals are not suffering from acute infection. However, this problem can be satisfactorily corrected by simultaneously detecting IgG and using quantitative detection reagents.
In the case of "2", if the initial test result is IgM positive /IgG negative, and the second test is carried out after 15 days -1 month, the individual will still keep this situation or IgM will turn negative, that is, IgG will not undergo serological conversion; If the initial test result is IgM positive or/and IgG positive, the second test after 15 days -1 month will not change much, especially IgG will remain stable, because the individual has not really relapsed infection.
07. Why is TORCH screening in pregnancy time-sensitive?
Different virus screening targets have different requirements for screening gestational age. Without gestational age, screening results are often meaningless. Some viruses are meaningful for screening in the first trimester, while others are meaningful for screening in the third trimester.
08. Why is there no absolute reference value for antibody screening?
The immune response of human body to virus infection is different, and the antibody level varies greatly among individuals. TORCH virus infection is a maternal-fetal dynamic process, and there is no clear standard in each time period. The cut-off value of IgG/IgM concentration is an infection index, but it has limitations, and the change of individual concentration gradient is more clinical. For example, when IgG rises four times, it is often used as an indicator of virus recurrence or reinfection.
09. Why is quantitative analysis the best choice for TORCH screening?
1. The production of IgG or IgM during pregnancy is a process of rapid change, which can only be detected by quantitative analysis of concentration changes.
2. Quantitative analysis is helpful to find false positive or false negative results.
3. For those pregnant women who haven’t made basic immune status assessment before pregnancy, we can detect the concentration (C1, C2) of IgG or IgM at two time points (T1, T2) and calculate the gradient of concentration change in unit time, which can effectively find the specific immune response caused by virus attack, but there is no reference value at present, and C2/C1 is more than 4 times. All these must be based on quantitative detection.
Second, cytomegalovirus (CMV) screening
10. Why should I do CMV screening during pregnancy?
CMV is the most common cause of intrauterine infection, with an incidence of 0.2%-2.2% in live births. Infection is one of the common causes of sensorineural hearing loss and mental retardation.
Screening pregnant women can help doctors understand their immune status, provide health education for pregnant women with negative IgG to prevent infection during pregnancy, and conduct dynamic monitoring and observation to prevent primary infection. Monitoring the viral DNA replication of IgG positive pregnant women in the first and third trimester to prevent recurrent infection from causing congenital infection through placenta and neonatal infection through birth canal or milk.
Although the mother is immune (IgG positive), the baby may suffer from congenital CMV infection. Both the mother’s initial infection and recurrent infection can lead to symptomatic CMV infection in the fetus. Some immune mothers have congenital CMV infection in their babies, which leads to central nervous system damage.
Screening pregnant women can help doctors diagnose the infection types of pregnant women, so as to choose an effective time for prenatal diagnosis of the fetus. Do early detection and early intervention.
11. How to diagnose the first and recurrent CMV infection during pregnancy?
After the human body is infected with CMV for the first time, the virus enters a dormant period and lurks in the human body. This virus can be reactivated in human body, which is called recurrent (secondary) infection. In addition, individuals with immunity can be reinfected when exposed to exogenous new virus strains. Therefore, recurrence or reinfection is defined as the intermittent secretion of virus under the immune state of the host. This may be due to the reactivation of endogenous virus or the exposure of host to exogenous new virus strains.
Recurrent infection and reinfection can not be distinguished by serological detection, but only by virus isolation and molecular biological detection. One-third of healthy women with CMV IgG positive in the United States will be infected with the new CMV virus strain again within three years.
Serological diagnosis of CMV primary infection during pregnancy;
1. IgM positive+IgG quantitative detection rose, turned positive after 15 days, and seroconversion occurred = primary infection.
2. IgM positive+IgG low affinity (≤ 16 weeks) = first infection. If the immune status before pregnancy is unknown, the diagnosis of the first infection should be based on the detection of specific IgM antibody. However, IgM antibodies can also be detected in 10% cases of recurrent infection, and IgM antibodies can also be detected in serum several months after the first infection.
Therefore, IgM antibody positive people may include two situations: first infection before pregnancy and recurrent infection, as well as false positive caused by long-term IgM antibody positive. Affinity analysis of IgG antibody can help to know whether CMV infection occurs within 3 months, that is, if the test result is high affinity, it can be concluded that the first infection occurred 3 months ago, which often indicates previous infection; If the test result is low affinity, it is very likely that CMV will be infected for the first time within 3 months, which often indicates acute primary infection.
The detection of virus DNA can help us to find virus replication, but we can’t distinguish between recurrent infection and primary infection. Only those with positive IgG antibody can have recurrent infection.
Usually avidity index < 30%, it is highly suggestive of the recent first infection (within 3 months). Therefore, the serological diagnosis of the first infection in pregnancy is mainly based on the phenomenon of seroconversion (specific IgG antibody appeared in pregnant women with negative seroreaction before), or the detection of virus-specific IgM antibody accompanied by low affinity IgG antibody. If pregnant women detect IgG antibodies in pre-pregnancy serum without IgM antibodies, but there is a significant increase in IgG antibody titer and high IgG affinity (with or without specific IgM antibodies) during pregnancy, it can be considered as recurrent infection.
Diagnosis of non-primary infection (recurrence and reinfection) in pregnancy;
1. IgM positive+IgG positive+high affinity (≤ 16 weeks) = the possibility of non-primary infection increases.
2. IgG positive and IgM positive/negative+high affinity (≤ 16 weeks)+CMV (virus isolation or PCR) detected in urine/secretion/blood = non-primary infection.
3, CMV specific IgG increased by 4 times = non-primary infection.
12. What is CMV congenital infection? What are the consequences?
Congenital infection is caused by vertical transmission of CMV through placenta. The primary or secondary infection of pregnant women may be transmitted vertically to the fetus. After the initial infection, the probability of intrauterine vertical transmission during pregnancy is 30%-40%, while the probability after recurrent infection is only 1%.
However, the statistical analysis of fetal congenital defects published by CDC in 2009 showed that the CMV infection rate was 75% during pregnancy, and the congenital defects caused by CMV infection accounted for the first place.
Among them, the recurrence of CMV infection during pregnancy is the main factor, especially for pregnant women in China whose pre-pregnancy infection rate is as high as 95%. 10%-15% of infants with congenital infection will have symptoms at birth, including intrauterine growth retardation, microcephaly, hepatosplenomegaly, ecchymosis, jaundice, chorioretinitis, thrombocytopenia and anemia.
20%-30% of these babies will die, which is mainly due to disseminated intravascular coagulation, abnormal liver function or repeated bacterial infection. Most infants with congenital CMV infection (85%-90%) will not have symptoms or signs at birth, but 5%-15% of these infants will have sequelae, such as sensorineural hearing loss, psychomotor retardation and visual impairment.
13. How to make prenatal diagnosis of fetal CMV infection?
The diagnosis of fetal CMV infection should be based on the culture of amniotic fluid samples and PCR detection. When pregnant women are diagnosed with CMV infection for the first time, amniocentesis should be performed to collect amniotic fluid for real-time quantitative PCR detection after 7 weeks of maternal infection and 20-21 weeks of pregnancy, because only after 5-7 weeks of fetal infection, the amount of virus secreted into amniotic fluid can reach the detection limit through renal virus replication. According to many previous literature reports, if the time of prenatal diagnosis is too close to the time of maternal infection, the risk of false negative results can not be ignored.
There is no agreement on whether to take amniotic fluid virus test for cases with recurrent infection (the risk of fetal infection is low). However, according to literature reports, some cases of recurrent infection will also have serious sequelae. Therefore, even for cases of recurrent infection, we can consider prenatal diagnosis of fetal CMV infection through amniocentesis.
14. Why is it not recommended to diagnose fetal infection by detecting IgM antibody or DNA in fetal blood?
Not only because of the high risk of umbilical cord puncture, but also because many fetuses infected with CMV will have specific IgM antibodies only in the third trimester of pregnancy, which makes the sensitivity of umbilical cord puncture detection very low.
The sensitivity of fetal blood IgM is 50%-80%, the sensitivity of fetal blood DNA is 40%-90%, and the specificity and accuracy of amniotic fluid DNA can reach 100%.
15. Why is it not recommended to use CMV-DNA in pregnant women’s blood to detect primary infection?
Because the positive rate of CMV-DNA in the blood of pregnant women infected for the first time is 33.3%, and the positive rate of CMV-DNA in the blood of healthy women with IgG is 33.3%. Therefore, it is not reliable to diagnose infection with CMV-DNA in pregnant women’s blood.
16. What should I do after the fetus is diagnosed with CMV infection?
Because the prenatal diagnosis of CMV infection is limited to amniotic fluid detection (such as virus isolation and PCR), it is impossible to predict whether the fetus will have symptoms at birth. Therefore, once the fetal infection is diagnosed, pregnant women should have a series of ultrasound examinations every 2-4 weeks to find the signs of CMV infection, such as intrauterine growth retardation, ventricular dilatation, microcephaly, intracranial calcification, ascites or pleural effusion, fetal edema, oligohydramnios or excessive amniotic fluid, and enhanced intestinal echo. These findings may be helpful to predict the prognosis of the fetus.
The ultrasonic examination of this system should be carried out in a qualified prenatal diagnosis center. Fetal high-resolution magnetic resonance imaging may be helpful to evaluate the prognosis, especially when ultrasound finds brain abnormalities.
However, whether magnetic resonance imaging can provide us with effective information about fetal CMV infection remains to be further determined. Some studies have reported the clinical significance of viral load in amniotic fluid as a prognostic indicator. Studies have shown that the CMV-DNA load in amniotic fluid samples is significantly higher than that in asymptomatic fetuses.
However, there is a great deal of overlap between the two groups. Therefore, whether the quantitative determination of CMVDNA in amniotic fluid can be used as a prognostic indicator of CMV infection needs further confirmation.
Third, rubella virus (RV) screening
17. Why do you want pregnant women to receive antibody testing before pregnancy?
Rubella, also known as German measles, is a childhood disease. Non-pregnancy usually manifests as a slight self-limiting disease. However, the virus may have a destructive effect on the developing fetus during pregnancy, which is directly related to unpredictable abortion and serious congenital malformation.
Before pregnancy, we can know whether the body is immune to RV. If there is no immunity (IgG negative), we can vaccinate and get pregnant after producing antibodies. Vaccination or natural immunity can generally protect the fetus from intrauterine infection.
18. What is congenital rubella syndrome (CRS)? What are the most common congenital defects and delayed symptoms?
CRS represents the symptoms of newborns infected with RV before birth, and the fetus is involved in multiple organ systems. RV is harmful to developing fetus through vertical infection of placenta, which leads to spontaneous abortion, fetal infection, stillbirth and fetal growth retardation. Most children with CRS show persistent neuromotor defects, and pneumonia, thyroid dysfunction and progressive panencephalitis may occur in the future. The most common congenital defects and subsequent symptoms are shown in Table 3.
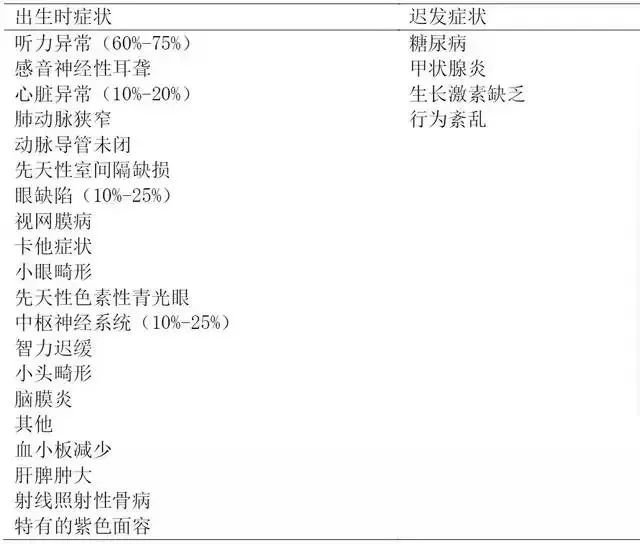
Table 3 Birth symptoms and delayed clinical manifestations of rubella virus infection
After RV infects placenta, it spreads through the vascular system of developing fetus, causing vascular cell disease and ischemia of developing organs. Fetal infection rate and birth defect rate are related to the gestational age at the time of maternal infection, as shown in Table 4.
When maternal infection/exposure occurs in the first trimester of pregnancy, the fetal infection rate is about 80%, which decreases to 25% in the second trimester, and then increases in the later trimester, from 35% in the 27th-30th week of pregnancy to almost 100% after 36th week of pregnancy. The infection rate of congenital defects was 90% before the 11th week of pregnancy, 33% at the 11th-12th week of pregnancy, 11% at the 13th-14th week of pregnancy, 24% at the 15th-16th week of pregnancy, and 0 after the 16th week of pregnancy.
Therefore, the risk of birth defects after maternal infection is limited to 16 weeks before pregnancy, and the risk of CRS caused by infection after 20 weeks of pregnancy is very small. The only sequela of infection in the third trimester may be fetal growth retardation (FGR). Maternal infection before and after fertilization does not increase the risk of CRS. Therefore, the consultation on fetal risk and management must be individualized.
Vaccination or naturally acquired maternal immunity can generally protect the fetus from intrauterine infection, but there are also reports of maternal re-infection with CRS. Therefore, the fetus or newborn with clinical symptoms of congenital infection should consider the possibility of CRS. No case of CRS was reported when the mother reinfected after 12 weeks of pregnancy.

Table 4 Relationship between rubella virus infection during pregnancy and fetal infection and birth defects
19. How to diagnose maternal RV infection?
Accurate diagnosis of primary RV in pregnancy is necessary, and serological tests are needed, because many cases are subclinical. It is a simple, sensitive and accurate method to determine RV-specific IgG and IgM by serological method. The diagnosis is as follows:
1. The titer of RV IgG antibody in acute and convalescent serum samples increased by 4 times.
2. RV specific IgM antibody was positive. IgM positive in pregnant women’s blood also needs serological conversion index, that is, IgG changes from negative to positive. Or pregnant women’s blood IgM(+) and IgG antibody appear at the same time, and the continuous double serum appears 4 times higher (between 15 days and 1 month).
3. RV culture is positive (virus isolation and culture of patients’ clinical samples). The serological test should be performed within 7-15 days after the appearance of the rash, and repeated after 2-3 weeks.
20. Can fetal RV infection be diagnosed?
There is no mature and stable diagnosis method at present. There are few reports that RV-specific PCR is used to detect CVS samples for prenatal diagnosis of intrauterine RV. The report confirmed that the chorionic villi sample is superior to the amniotic fluid sample, because chorionic villi can be taken at the first 10-12 weeks of pregnancy, while amniotic fluid needs to be taken at the 18th-20th week of pregnancy, and umbilical cord blood needs to be taken at the 28th week of pregnancy. At this time, it is of little significance to detect fetal infection.
Ultrasonic diagnosis of CRS is extremely difficult. Biometrics is helpful to diagnose FGR, but it is not a good tool to diagnose CRS. Because the malformations caused by RV are different, the fetus with growth retardation should consider whether there is congenital virus infection, including RV.
21. Pregnant women have signs or symptoms of rubella-like diseases. What should I do if I am exposed to RV recently?
When pregnant women are exposed to RV, they must be individualized according to their gestational age and immune status. It is often difficult to diagnose acute RV infection in pregnant women, and the clinical diagnosis is unreliable. A large number of cases are subclinical, and the clinical characteristics are very similar to other diseases. Fig. 3 shows the treatment guidelines for pregnant women who are exposed or have symptoms similar to rubella. If pregnant women have symptoms similar to rubella or have been exposed to RV recently, the gestational age and immune status should be determined.
It is difficult to diagnose pregnant women who have been exposed to RV for 5 weeks or have a rash for 4 weeks. If IgG antibody is negative, then the patient is sensitive to RV. Therefore, there is no evidence of recent infection. If IgG antibody is positive, it means that there was infection before. At this time, the antibody level is low, suggesting that it is a long-term infection. However, it is difficult to determine the infection time and the risk of fetal infection. It is recommended to determine IgM or repeat IgG antibody to see if there is a significant increase or decrease.
22. Can I be vaccinated when I am pregnant? Do you need to terminate your pregnancy if you accidentally vaccinate in the first trimester or if you are pregnant after vaccination?
Contraindications for rubella vaccination are fever, immunodeficiency, neomycin allergy and pregnancy. Rubella vaccine virus may infect fetus through placenta. However, the cases of CRS in the offspring of pregnant women who were accidentally vaccinated with rubella vaccine in the first trimester have not been reported.
Therefore, termination of pregnancy is not recommended for such pregnant women. According to the potential risk of the vaccine to the fetus, it is recommended that women get pregnant 28 days after vaccination.
23. Why only screen RV IgM antibody during pregnancy?
Will get the wrong result?
Because there will be non-acute infection positive, there are two main reasons:
True positive
Patients have been expressing IgM for many years, and the IgM level often remains at a low and stable level, often accompanied by IgG positive.
false positive
The specificity of detection methods is limited. The reference range of RV IgM antibody is determined by known negative and positive samples. As shown in Figure 4, the area below the red line is an overlapping area, which can’t be explained by reference method. About 3%-5% of IgM false positives will occur, and we can’t distinguish between true and false positives without quantitative methods. The main reasons for this situation are RF interference, cross reaction, polyclonal stimulation and so on.
24. What should I do if IgM antibody is positive during rubella virus screening during pregnancy?
Screening IgM antibody positive for rubella virus during pregnancy can be handled according to the flow chart in Figure 5.
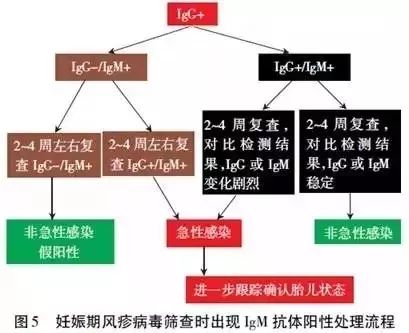
25. Why is prevention the best strategy to avoid CRS?
RV infection in pregnant women has a destructive effect on the developing fetus. The key to prevention is universal vaccination for all infants and immunization for high-risk groups in pre-pregnancy screening women. Try to diagnose the infected cases as much as possible. Avoid contact with RV as much as possible in the early and middle pregnancy, including IgG positive pregnant women.
For primary infections that occur before 16 weeks, pregnant women should be advised of the risk of vertical transmission and suggestions for termination of pregnancy. However, there is no intrauterine treatment for infected fetuses. Therefore, prevention is still the best strategy to avoid CRS.
Fourth, Toxoplasma screening
26. Why should pregnant women be screened for Toxoplasma infection?
1. Acute infection of Toxoplasma during pregnancy can seriously affect the health of fetus and newborn. If you ingest contaminated undercooked meat or eat contaminated food and water, it can lead to acute Toxoplasma infection.
2. Fetal toxoplasmosis is almost always caused by the primary infection of pregnant women, which can lead to fetal visual and auditory loss, mental and psychomotor development retardation, seizures, abnormal blood system, hepatosplenomegaly, and even death.
3. Toxoplasma infection to the fetus often occurs when pregnant women have no medical history during pregnancy, have not eaten uncooked meat, and have not been exposed to cats. Therefore, the determination of whether to carry out serological testing for pregnant women can not only rely on clinical (such as the presence or absence of symptoms) or epidemiological conditions (such as contact with Toxoplasma gondii).
Systematic education and serological examination for pregnant women are effective methods to prevent infection, diagnose and treat fetal infection early, because Toxoplasma infection is usually hidden. Practice has proved that the treatment of fetuses and infants under one year old is the most effective for improving clinical symptoms. All pregnant women should undergo serological examination of IgG and IgM antibodies against Toxoplasma gondii as soon as possible (ideally in the first three months of pregnancy).
27, fetal infection with Toxoplasma gondii is the first infection or recurrent infection caused by pregnant women?
Fetal infection occurs mainly because pregnant women are infected with Toxoplasma gondii for the first time during pregnancy. In a few cases, because pregnant women have chronic infection, due to autoimmune deficiency (such as AIDS patients or patients treated with corticosteroids), Toxoplasma activation occurs, resulting in congenital infection.
28. What is the relationship between fetal and neonatal infection and maternal infection?
With the increase of gestational age, the possibility of vertical transmission also increases, as shown in Table 5. The clinical symptoms of infected newborns of pregnant women infected in early pregnancy are more serious, as shown in Table 5.

29. Which pregnant women are high-risk groups of Toxoplasma infection? How can we be sure?
Detection of Toxoplasma IgG negative or IgM negative before and during pregnancy shows that the woman has not been infected by Toxoplasma gondii as a high-risk group for the first infection during pregnancy, and there is a risk of acquiring the first infection and transmitting it to the fetus during pregnancy. Therefore, serological examination of IgG and IgM antibodies for high-risk women during pregnancy is supported.
For women with negative serological examination, it is best to check once a month in the first trimester and once every three months thereafter. It is necessary to identify high-risk screening in the early pregnancy, and the earlier the better, the greater the help it will provide to the clinic. The test results obtained in the second trimester often cannot determine whether there is infection in the first trimester.
30, toxoplasmosis during pregnancy screening process and clinical explanation?
1, regarding the disposal of toxoplasmosis, you can consult an expert during pregnancy.
2. Consider sending the sample to the reference laboratory.
3. Treat with acetylspiramycin or pyrimethamine, sulfadiazine and formyltetrahydrofolate.
4, amniotic fluid PCR detection should be in the 18th week of pregnancy (not in advance) or after 18 weeks. When the gestational age is more than 18 weeks, the risk of detection and the benefit of diagnosis of fetal infection should be carefully weighed.
5. The screening and treatment process of toxoplasmosis during pregnancy is shown in Figure 6, and the clinical explanation is shown in Table 6. In the United States, it can reduce the unnecessary abortion of IgM positive pregnant women by 50%.
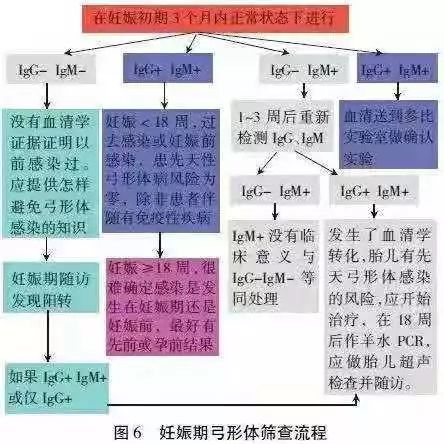
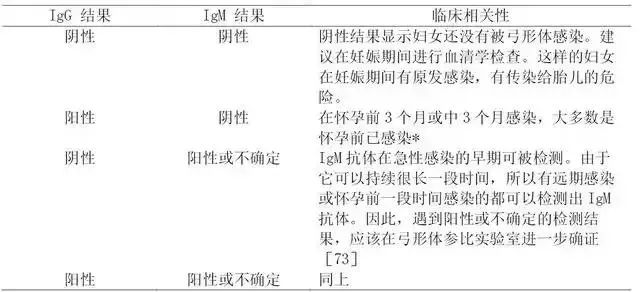
Table 6 Interpretation of serological test results of Toxoplasma gondii in clinical (non-reference) laboratory
Note: * It is not easy to explain this situation in the last three months of pregnancy. Although this situation is consistent with pre-pregnancy infection, in some patients, this result can be reflected in the early pregnancy infection, which is usually accompanied by the increase of IgM antibody titer and the decrease of detection limit in a relatively short period of time.
31. Whether IgM antibody is positive before or during the second trimester of pregnancy, it cannot be considered as a recent infection?
A positive IgM antibody result cannot be considered as a recent infection. IgM antibody in acute infection can exist for more than one year, and most pregnant women with positive IgM antibody have been infected for a long time, which has exceeded the period of affecting the fetus.
These patients are chronically infected. Jose continuously detected 100 serum samples which were sent to PAMF-TSL reference laboratory after being tested positive for IgM antibody in other laboratories. Confirmatory experiments in PAMF-TSL reference laboratory confirmed that 62% of these serum samples were negative for IgM antibody.
Additional experiments confirmed that these infections were long-term rather than recent. The greatest value of IgM antibody positive test results is that it emphasizes the possibility of recent infection, so it needs to be further confirmed in reference laboratories.
32. How many possibilities are there for the final interpretation of Toxoplasma antibody test results in reference laboratories?
There are three possibilities for the final interpretation of serological test results in reference laboratories:
1. The test results are consistent with the recent infection, suggesting that the patient acquired infection after pregnancy, but it cannot be ruled out that the infection is about to become pregnant.
2. The test results are consistent with the long-term infection before conception.
3. The test results are uncertain, and further serological tests are needed for parallel detection.
33. What is the clinical significance of Toxoplasma IgG affinity test?
Toxoplasma IgG affinity test is generally used in reference laboratories. After infection, high affinity IgG antibody does not appear until at least 12-16 weeks of pregnancy (the specific time depends on the experimental method used). The appearance of high affinity antibodies suggests that the infection occurred at least 16 weeks ago. Therefore, regardless of the results of IgM antibody test, the results of high affinity IgG test suggest that the fetus will not be infected with congenital Toxoplasma gondii basically.
For pregnant women who are more than 16 weeks pregnant, the results of high affinity experiment are very useful to determine that the infection is at least before the first 12-16 weeks of pregnancy. In this case, compared with the infection in the third trimester, the rate of Toxoplasma infection to the fetus is very low, but the possibility of fatal damage to the fetus is very high, as shown in Table 5, and the negative predictive value of amniotic fluid PCR is very high, as shown in Table 7.
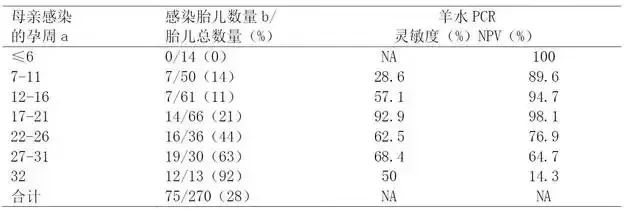
Table 7 Relationship between gestational age of maternal infection, fetal infection and amniotic fluid PCR
Note: No matter how many weeks you are pregnant, the positive predictive value is 100%, and the data comes from the literature. NA: Not applicable. A: 270 pregnant women were detected by seroconversion method to determine maternal infection, of which 261 cases (97%) were treated with acetylspiramycin. B: After one year, the congenital infection was diagnosed by the existence of IgG antibody against Toxoplasma gondii.
It is important to note that after the initial infection, low affinity or uncertain test results may exist for several months or more than one year. For this reason, Toxoplasma IgG affinity test cannot be used alone to determine whether it is a recent infection. In fact, if there are antibodies with low affinity or critical affinity in serum samples, IgM antibody test results are negative, suggesting long-term infection. If used alone, IgG affinity test is useless and may even be misleading.
34. How to diagnose fetal infection if toxoplasmosis is suspected for the first time during pregnancy?
The PCR amplification of DNA of Toxoplasma gondii in amniotic fluid at 18 weeks (optimal time) or later stage has been successfully applied to prenatal diagnosis of congenital Toxoplasma gondii. Amniotic fluid examination in the early pregnancy is of high risk to the fetus and unnecessary. The data showed that the detection at 17-21 weeks of pregnancy had the highest sensitivity and the best negative predictive value (the positive predictive value was 100% no matter how many weeks of pregnancy).
Ultrasound is mainly used for pregnant women who are suspected or diagnosed with acute infection within a short time (3 months) before pregnancy. Ultrasound can show fetal malformations, including brain edema, brain or liver calcification, splenomegaly and ascites.
Question 36: When did acetylspiramycin start to treat toxoplasmosis during pregnancy?
Once acute infection is suspected, and the infection occurs within 18 weeks of pregnancy or a short time before pregnancy, some scholars in the United States and Europe think that acetylspiramycin is the first choice to prevent the vertical transmission of parasites.
35. When should pyrimethamine, sulfadiazine and formyltetrahydrofolate be combined to treat Toxoplasma infection?
If the positive result of amniotic fluid PCR confirms fetal infection, pyrimethamine, sulfadiazine, formyltetrahydrofolate, etc. are recommended at or after the 18th week of pregnancy (if the patient has already applied acetylspiramycin, it is recommended to change to combined medication). Pyrimethamine has teratogenic effect, so it can’t be combined with drugs before the 18th week of pregnancy (in some centers in Europe, combined drugs can be used as early as the 14th-16th week of pregnancy).
Due to the high proportion of vertical transmission after 18 weeks of pregnancy, if Toxoplasma gondii is infected after 18 weeks of pregnancy, pyrimethamine, sulfadiazine and formyltetrahydrofolate are also recommended to prevent fetal infection.
If vertical transmission has occurred, prepare to treat the fetus. At this time, pyrimethamine can not be used early because it has potential teratogenic effect. Acetylspiramycin, a macrolide antibiotic, can reduce the probability of vertical transmission. However, well-designed research has not been carried out. It has been reported that this protection is effective for early pregnancy infection.
According to the historical comparison, the probability of congenital infection was reduced by about 60%. Acetylspiramycin can’t pass through the placenta quickly, so the therapeutic effect on fetal infection will not be very good. There is no evidence that acetylspiramycin has teratogenic effect, as shown in Table 8.
Because in theory, the infection of pregnant women in the first trimester can be transmitted to the fetus in the third trimester, so even if the PCR result of amniotic fluid is negative, acetylspiramycin should always be used for delivery. Pregnant women with high risk of fetal infection, or fetal infection has been confirmed. After 18 weeks of pregnancy, the treatment scheme of acetylspiramycin alone should be changed to the combination of pyrimethamine, sulfadiazine and formyltetrahydrofolate. Medication must be applied under the guidance of medical experts.
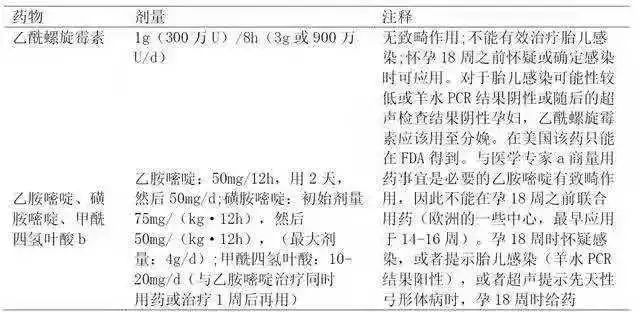
Table 8 Drug use of pregnant women suspected or confirmed to be infected with Toxoplasma during pregnancy
Note: FDA: US Food and Drug Administration.
a:PaloAltoMedicalFoundationToxoplasmaSerologyLaboratory,telephonenumber(650)853~4828,orUS(Chicago,IL)NationalCollaborativeTreatmentTrialStudy,telephonenumber(773)834~4152.
B: Folic acid cannot be used as a substitute for formyltetrahydrofolate.
36. Do you still need prenatal diagnosis and anti-Toxoplasma treatment if you have been infected with Toxoplasma before pregnancy (IgG antibody positive)?
If Toxoplasma gondii is definitely infected before pregnancy or serological tests show that Toxoplasma gondii was infected a long time ago (before pregnancy), the probability of children suffering from congenital Toxoplasma gondii is very small (close to zero), so it is not necessary to apply acetylspiramycin or pyrimethamine, sulfadiazine and formyltetrahydrofolate for treatment and prenatal diagnosis of fetal infection unless the mother’s immune function is defective.
37. What information can be obtained from each suspected toxoplasmosis case through consultation and discussion with experts?
1. Selection of detection methods for Toxoplasma gondii.
2. Correct interpretation of test results.
3. Prenatal diagnosis of pregnant women and fetuses.
4. Give an individualized treatment plan.
38. How to deal with patients with immune deficiency who have been infected with Toxoplasma before pregnancy?
Pregnant women infected with HIV and toxoplasmosis are at risk of toxoplasmosis reactivation and development, such as toxoplasmosis encephalitis and pneumonia, and/or transmission of parasites to offspring.
Pregnant women infected with HIV are not suitable for amniotic fluid PCR detection, because it is possible to transmit HIV to the fetus during amniotic fluid puncture.
Pregnant women who are not infected with HIV and are chronically infected with Toxoplasma gondii with immunodeficiency (or infected during pregnancy) may consider amniotic fluid PCR detection. For all pregnant women with chronic toxoplasmosis and immunodeficiency, ultrasound examination should be done once a month.
39, recently infected with Toxoplasma gondii, when is it safe to get pregnant?
After women of childbearing age are clearly infected with Toxoplasma gondii, considering the risk of transmission to future generations, they often ask them when it is safe to get pregnant. There is no exact information about this problem at present. Conservative advice is that such women wait six months (from the time when the acute infection is diagnosed or recorded) before getting pregnant. It is best for every patient to consult an expert.
V. Screening of herpes simplex virus (HSV)
40. What is the purpose of screening for herpes simplex virus (HSV) before and during pregnancy?
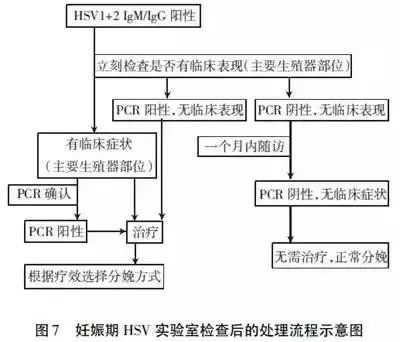
Serological screening of HSV during pregnancy can determine whether pregnant women are infected with the virus. The comparative screening of HSV before pregnancy and during pregnancy is to determine the first infection or recurrent infection, so as to provide the basis for treatment and intervention, with the aim of preventing neonatal infection. The treatment process after laboratory examination during pregnancy is shown in Figure 7.
41. What are the characteristics of HSV infection during pregnancy?
1. Once infected and carried for life, the virus lurks in the ganglion, which is a kind of herpesvirus.
2. The probability of fetal infection caused by primary infection in early and middle pregnancy is extremely low, and screening in early pregnancy is of little significance. It is rare for HSV to cause fetal malformation through blood-placenta, such as microcephaly, hepatosplenomegaly, intrauterine fetal death (IUFD) and intrauterine growth retardation (IUGR).
3. HSV infection mainly infects newborns through birth canal.
4. HSV infection during pregnancy is mainly the first infection. Previously, IgG positive had a weak protective effect on neonatal HSV infection.
5. neonatal infection rate: the first infection of pregnant women > recurrent infection.
6. Infants are infected with HSV during delivery, and 60%-80% of them have no history of genital herpes infection or sexual partners have no history of genital herpes before delivery.
7. 70% of mothers didn’t know they were infected until they found their newborn infected.
8. Both HSV-1 and HSV-2 can cause genital herpes infection (up to 50% of the initial infection of genital herpes is caused by HSV-1, but the virus shedding of recurrent and subclinical infections is mainly caused by HSV-2). Whether HSV-1 or HSV-2 is infected for the first time in the third trimester of pregnancy, the risk of infection to newborns is 30% ~ 50%.
42, neonatal HSV infection? Congenital HSV
Neonatal HSV infection refers to the infection acquired by the newborn through contact with maternal reproductive tract virus near delivery or delivery. In rare cases, iatrogenic infection may also occur or the virus may be infected through the baby’s mouth or broken skin after delivery. The definite diagnosis of HSV infection needs to be 48 hours after the end of delivery, which is very important for distinguishing newborns from congenital HSV infection. Congenital HSV infection is rare, and HSV infects the fetus through the placenta in utero.
43. How many kinds of genital herpes infections are there?
The first infection, the patient is exposed to HSV-1 or HSV-2 virus for the first time, and has not been exposed to either or both viruses before, and has no immunity (no HSV-1 or HSV-2 antibody).
It is not the first attack of the initial infection, and the patient is clinically diagnosed with HSV infection for the first time, but the corresponding antibodies have been produced in the body during the previous infection of HSV-1 or HSV-2 virus.
Recurrence of symptoms refers to the obvious clinical manifestations, the confirmed patient infected with HSV virus and produced antibodies, and then relapsed.
44, HSV serological detection should pay attention to what problems?
The following questions should be paid attention to in serological testing:
1. It is suggested that all pregnant women suspected to have HSV infection should use the method of direct virus detection (I-A). However, the results of direct virus detection for those with a history of recurrent infection or atypical reproductive tract diseases can be negative (Ⅲ, b).
2. HSV-2 antibody can diagnose genital herpes; HSV-1 antibody can’t distinguish between genital tract infection and oropharyngeal infection.
3. HSV-2 IgG is negative and HSV-1 IgG is positive, which should be considered as an uncommon recurrent reproductive tract infection.
4, the first genital herpes, should distinguish between the first infection and previous infection (Ⅲ, b). When symptoms appear, since IgG against HSV of the virus has not been produced (consistent with the type detected by genital lesions), evidence of seroconversion of primary infection should be found during follow-up.
5. The specific HSV antibody of primary infection can be detected 2 weeks to 3 months after symptoms appear. Where there are clinical symptoms, follow-up sampling should be taken to confirm serological conversion (IIa-B).
6. Because patients with early infection lack IgG antibody, detection of IgM antibody against herpes simplex virus can improve the detection rate of early infection (Ⅱb-B). However, because HSVIgM can be positive in recurrent infection and negative in primary infection, it is a non-viral specific antibody.
7. At present, there is no HSVIgM typing kit approved by FDA, and the truly reliable typing detection method for identifying acute HSV infection should be the molecular biological method of PCR technology.
45. How to deal with HSV primary infection in the third trimester of pregnancy?
In the third trimester of pregnancy, pregnant women are primarily infected with HSV, which has the most serious impact on newborns. In this case, because the mother can’t produce enough IgG antibodies before delivery, the baby lacks passive immune protection from maternal IgG. At this time, 30% ~ 50% of newborns will be infected with HSV.
46、How to deal with recurrent HSV infection in pregnant women during delivery?
When HSV is found to have recurrent infection, precursor symptoms or lesions during delivery, doctors should recommend parturient to choose cesarean section, even if the lesions are far away from vulva (such as buttocks or thighs), because both cervix and vagina may secrete viruses. Ideally, in order to prevent infection, cesarean section should be performed within 4 hours of rupture of the membrane.
If delivery has started, choosing cesarean section is likely to have no help in reducing infection rate. On the other hand, the protective effect of cesarean section has not been confirmed when vaginal HSV replication is active and fetal membrane rupture is delayed.
47. Is the serological typing of HSV in pregnancy clinically significant?
Both HSV-1 and HSV-2 can produce herpes in genital area and can cause neonatal infection. At present, the clinical treatment strategy will not be different because of the classification, nor will it be changed according to the different parts of HSV infection or different tissues and organs. Moreover, the proportion of HSV-1 type in genital herpes is also very high, reaching 1/3.
Therefore, typing detection is not necessary. Of course, typing detection can provide some reference information, but typing with PCR technology is the most reliable.
48. How to deal with the recurrent HSV infection during pregnancy?
When pregnant women with pregnancy time less than 36 weeks have recurrent HSV infection, antiviral treatment should not be used even if the clinical symptoms are serious. When the clinical symptoms are very serious and antiviral treatment is unavoidable, individualized treatment should also be considered. When the pregnancy time is more than 36 weeks, antiviral therapy can reduce its infectivity, relieve clinical symptoms and reduce the rate of cesarean section.
In the absence of a large number of clinical safety research data, pregnant women can only use acyclovir and valaciclovir when there are indications for antiviral treatment.
49. Do women need serological examination without a history of HSV infection?
Infants are infected with HSV during delivery, and 60%-80% of them have no history of HSV infection or sexual partners have no history of genital herpes before delivery. 70% mothers didn’t know they were infected until they found out that their newborn was infected.
50, the first genital herpes during pregnancy postpartum newborn how to deal with?
Postpartum newborns of pregnant women with genital herpes for the first time during pregnancy should be treated as follows:
1. First, the pediatrician should be informed.
2. In order to detect neonatal infection early, HSV culture of urine, stool, oropharynx, eyes and skin should be done.
3. Before the culture results come out, we should weigh the advantages and disadvantages and study whether to start acyclovir treatment.
4. If acyclovir treatment is not started immediately, the newborn should be closely monitored for signs of lethargy, fever, refusal to eat or pathological changes.
51. How to deal with postpartum newborns with recurrent genital herpes during pregnancy?
Although some doctors believe that pregnant women who have recurrent genital herpes during pregnancy should take a set of samples for virus culture after delivery, which is helpful for early detection of neonatal infection, there is no evidence to support this practice. However, doctors and parents should be advised to consider the differential diagnosis of HSV infection if the baby has any lesions of skin, eyes or mucosa, especially within 2 weeks of birth.
essay Source: Reproductive Medicine Center of the Second Affiliated Hospital of Heavy Medical College. If the subscription number is infringed or reprinted, please contact us, and we will contact you as soon as possible. Row deletion.
Editor: green,Review: Chenchen