Focus on Suzhou ADC pharmaceutical companies, who is the king of financing?
According to the statistics of the mainland headquarters of China ADC start-up pharmaceutical companies, it is found that Suzhou is the city with the largest number of distribution except overseas.
Taking this as the breakthrough point, the Intellectual Pharmacy Bureau conducted a panoramic analysis of Suzhou ADC start-up pharmaceutical companies, involving information such as financing rounds, total financing, investment institutions, company profile and clinical pipeline layout.
Due to different statistical caliber, if there are errors or omissions in the full-text data, please criticize and correct them.
1, the overall situation
According to the monitoring of the Intellectual Drug Administration, ADC start-up pharmaceutical companies distributed in SuzhouHas reached nine.They are: Kai Tak Medicine, Tongyi Medicine, Yilian Biology, Yingen Biology, Pufang Biology, Puling Biology, Laitekang Biology, Lianning Biology and Sidao Medicine.
And the location of the mainland headquarters of the above companies is all inSuzhou Industrial ParkThe park is a bio-pharmaceutical industrial park with domestic industrial competitiveness.
on the one handIt shows that the agglomeration effect of Suzhou ADC pharmaceutical companies is outstanding, forming a complete industrial chain from upstream target screening and confirmation, compound synthesis and screening to preparation production.
on the other handIt also shows that the fault phenomenon of medical development in Suzhou is prominent, and Suzhou Industrial Park occupies the dominant position.
It is reported that the total amount of biomedical financing in Suzhou Industrial Park in 2022 accounts for the total amount of financing in Suzhou.53%.

Why Suzhou Industrial Park?
One isSuzhou, with its back to Shanghai, is influenced by Shanghai’s radiation. However, compared with Shanghai, Suzhou’s operating costs in terms of venues, materials and personnel are significantly lower than Shanghai’s, which has great attraction to many biomedical enterprises, especially small and medium-sized biomedical enterprises, and is also the place where Shanghai’s biomedical industry is transferred.
The second isSuzhou is located in the Yangtze River Delta, with developed economy, strong residents’ consumption power and large population density, and is a domestic pharmaceutical consumption market.
From the technical characteristics,With the development of dual reactance and ADC technology, more and more enterprises in Suzhou are developing.Double-impedance ADC.
Double antibody ADC combines the advantages of double antibody and ADC: compared with monoclonal antibody, it can target tumor cells more specifically, overcome drug resistance and increase drug safety.
For example,Tongyi Medicine and Sidao MedicineIt is an important participant in the dual-impedance ADC.
2. Overall investment situation
From the perspective of investment rounds, the overall capital is inclined to the early stage of the market, focusing onAngel wheel,A roundandB wheelOnly one company entered the C round, Kai Tak Medicine.
Judging from the disclosed financing amount, the amount is on the high side. Among them,Pufang biologyThe total financing amount is the highest, which is US$ 135 million, which is converted into RMB 964 million at the current exchange rate.
Followed by Yingen Bio and Yilian Bio, converted into RMB 858 million and RMB 850 million at the current exchange rate.
It is worth mentioning that in May this year, Tongyi Pharmaceutical announced the completion of the B/B+ round of financing of nearly 100 million US dollars. In the cold winter of capital, financing of nearly 100 million US dollars is really rare.
From the perspective of investors, on the one hand, in a single round of financing,There are many participating institutions.; On the other hand, due to the high investment threshold of ADC drugs, most of them areProfessional medical investment institutionIn the layout, such as Tonghe Yucheng, Tonghe Capital, Cailletet Capital, etc.
In addition, Yuanhe Holdings and Yuanhe Origin Investment Institution, which were hatched in Suzhou Industrial Park, are also accelerating their layout.
It is not difficult to guess that this is one of the new ways to attract investment in Suzhou. The purpose of attracting investment with capital is to promote the integrated development of "funds+talents+industries+projects".
butSequoia Capital and Lilly AsiaVenture capital institutions such as * continue to invest real money in Suzhou ADC pharmaceutical companies.
For example,Pufang biologyThe A+ round of financing was led by Sequoia China, followed by Lilly Asia Fund, Yuanhe Holdings, Changan Capital, Zhouling Capital and Xianfeng Qiyun.
3. Company profile and pipeline layout
Qide medicine
Kai Tak Medicine is the first in the world to devote itself to.Enzymatic fixed-point coupling techniqueOne of the pioneers in developing ADC drugs, iLDC? and iGDC?, a complete underlying technology system, can provide full-process solutions for all kinds of coupled drugs from molecular design to commercial production. Based on this technology platform, several innovative ADC varieties have been successfully developed in clinical stages.
Official website shows that Kai Tak Medicine currently has a total of10 clinicsThe project under research mainly involves the field of cancer, with GQ1001 as the fastest progress, and is currently undergoing global multi-center clinical trial development.
GQ1001 is an ADC drug for the treatment of HER2+ solid tumor, which is synthesized by site-specific conjugation of toxin DM1 and trastuzumab, based on GQ’s unique ligase-dependent conjugation and patented ring-opening ligation technology.
GQ1005 is an ADC drug targeting human epidermal growth factor receptor 2(her2), and it is planned to be developed to treat solid tumors such as breast cancer and gastric cancer with HER2 expression.
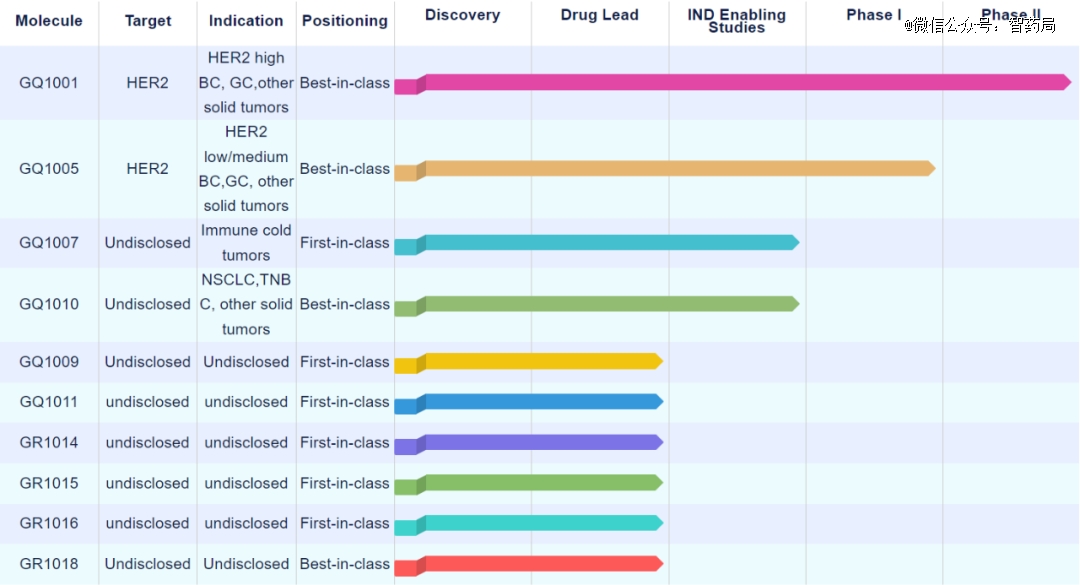
Figure: Layout of medical pipelines in Kai Tak
Tongyi medicine
Tongyi Medicine is a *.Dual-target ligand coupled drug bi-XDCInnovative pharmaceutical companies, based on the unique dual-target XDC technology, have established three core technology platforms, including BEST (bifunctional ligand-coupled drugs), C-PROTAC and chronic drug design technology.
Official website shows that the company currently has three products and seven clinical trials are conducted simultaneously.
Among them, CBP-1008 is a double-antibody adc developed by Tongyi Medicine, which targets frα and trpv6 (a calcium channel protein) receptors. Among them, frα is highly expressed in ovarian cancer, breast cancer and other tumor species, and trpv6 is highly expressed in breast cancer, pancreatic cancer and other tumor species. Phase Ib clinical efficacy has been confirmed, and phase II single-arm clinical trial is in progress.
CBP-1018 has basically completed the dose climbing in phase Ia and is in the stage of curative effect exploration. CBP-1019 has started phase I/II international multi-center clinical research.

Figure: Tongyi Pharmaceutical Pipeline
Yilian biology
Yilian Bio is a clinical biopharmaceutical company specializing in the development of innovative coupling drugs. It has developed the latest generation of novel antibody coupling drug platform technology of Tumour Micro Enviroment Activable Linker-Payload (Tmalin) with independent intellectual property rights, which can realize uniform and stable coupling with high DAR value, further improve the therapeutic window of ADC drugs and enhance the therapeutic effect of ADC drugs in solid tumors.
At present, seven clinical pipelines of Yilian Bio are generated based on its ADC technology platform TMALIN, which mainly involves the field of tumor, with the fastest progress being YL201 and YL202, all of which have entered clinical phase I.. <_o3a_p style="outline:0px;" >
YL201 is an adc targeting b7h3, which is composed of anti-b7h3 antibodies and drugs connected by a novel tripeptide linker. YL202 is an adc drug targeting her3, which is in the early clinical stage worldwide.
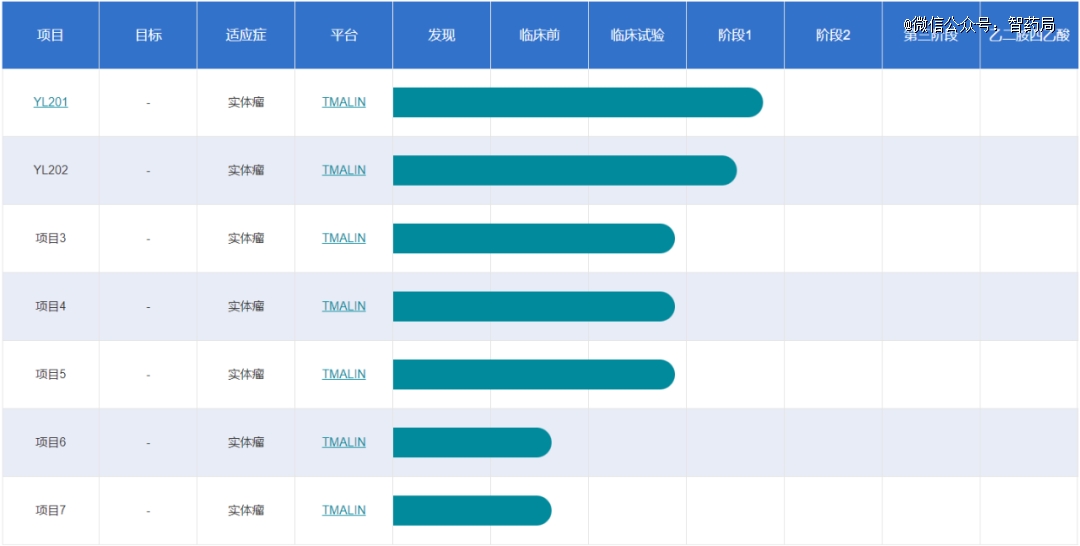
Figure: Yilian Biological Clinical Pipeline
Ying’ en biology
Yingen Bio is an innovative biopharmaceutical research and development company in clinical stage, focusing on developing antibody-coupled drugs for treating cancer and autoimmune diseases. Based on its sexDITAC and DIMAC platformsWe have developed more than 10 product lines of antibody drug conjugate (ADC) drugs of FIC and BIC.
In the research pipeline, DB-1303 is the most advanced product, which is a new generation of HER2 ADC developed by Yingen Bio. It consists of anti-HER2 monoclonal antibody, shearable linker and proprietary topoisomerase I inhibitor P1003.
It shows strong activity and bystander effect, good safety and wide treatment window in tumor models with positive and low expression of HER2.
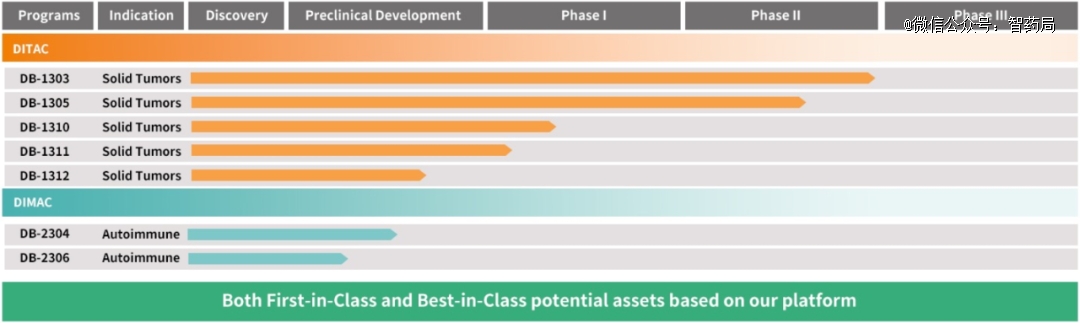
Figure: Ying ‘en Biological Clinical Pipeline
Pufang biology
Pufang Bio is a company that focuses on developing a new generation of macromolecular targeted drugs. Its product pipeline mainly includes antibody-coupled drugs (ADC) and other antibody-based therapies. Based on the innovative technology platform, a series of product lines composed of candidate drugs for various tumor targets have been developed, which are currently in the stage of drug discovery and preclinical development. Pufang Bio has branches in Greater Seattle, Washington, USA and Suzhou, China.
PRO1184 and PRO1160 are two adc drugs that Pufang Bio took the lead in clinical research. PRO1184 is a model based onFolate receptor α(FRα)A novel hydrophilic linker with independent patent was adopted as the antibody-coupled drug with exetecan as the target and the payload.
PRO1160 is an ADC targeting CD70, which is also made up of a new hydrophilic connector and exatecan, and has the potential to become a similar ADC therapy with a wide treatment window.
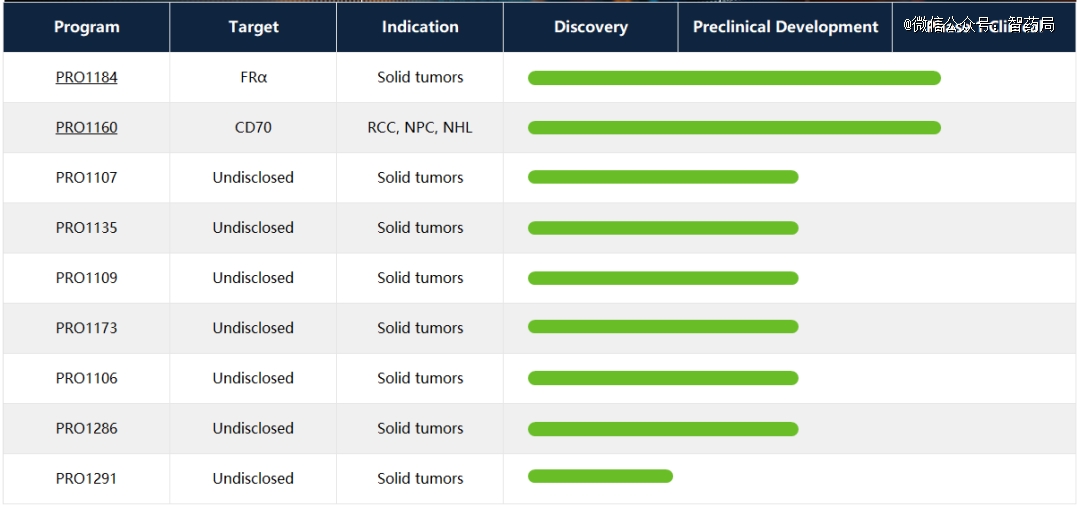
Figure: Pufang biological clinical pipeline
Puling biology
Puling Bio is an innovative ADC biotechnology company, which is committed to developing the next generation of accurate and flexible drug delivery systems. The founding team comes from listed ADC companies in the United States and has rich experience in the whole process of ADC development.
The company has established a flexible and modular technology platform to synthesize ADC drug pool, and screened * ADC drug composition for each target through a unique screening platform, and built a connector platform with independent intellectual property rights, and developed the "First in class & Best in class" product pipeline.
At present, Puling Bio has 8 pipelines under research, all of which are in preclinical stage, including 5 self-developed pipelines and 3 cooperative pipelines.
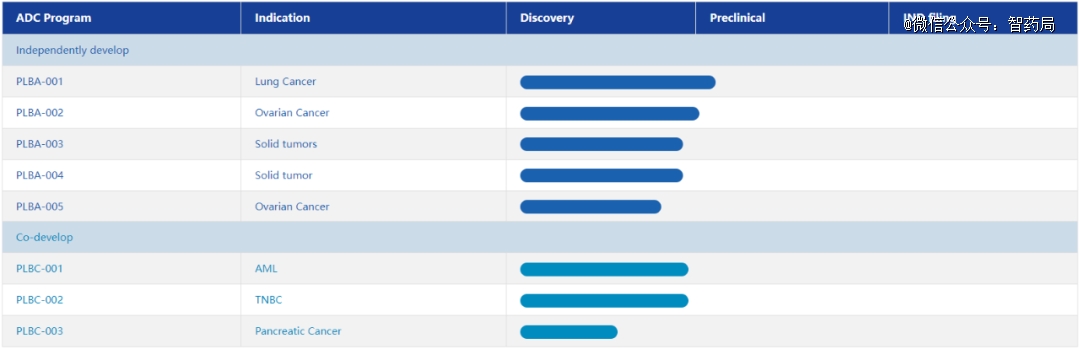
Figure: Puling biological clinical pipeline
Laitekang biology
Letcom focuses on the field of malignant tumors and autoimmune diseases, and develops innovative drugs with higher safety, better clinical efficacy and global intellectual property rights by using cutting-edge antibody technologies such as multifunctional antibody fusion protein, bispecific antibody and complementary bispecific ADC.
At present, the company has completed the conceptual design of more than 10 First in class drugs, 5 of the 7 initiated projects have successfully obtained antibody molecules, and 1 IND application has been submitted to the FDA. At the same time, the company has also actively accelerated the layout of core patents at home and abroad and built an intellectual property protection system for enterprise innovations.
Lianning biology
In addition to the traditional monoclonal antibody coupling, Lianning Bio has developed the business of double antibody coupling, polypeptide coupling, oligonucleotide coupling and nuclide coupling in parallel. Relying on the new generation of fixed-point coupling technology with independent intellectual property rights and the technical accumulation of ADC/Drug-Linker for many years, three coupling technology platforms with low toxicity to high toxicity have been established, which meet the needs of different targets and antibody drug formation.
Lianning Bio has completed the DMF filing of 10 small molecule products related to ADC drugs in the United States, including such common toxins as DUO-5, MMAE and Exatecan, as well as drugs-connectors such as VcMMAE, AS269, Gly-EDA-PNU and LND1025 which are directly applied to the ADC clinical production approval documents.
Sidao medicine
Xadcera is committed to developing globally competitive.Double-impedance ADCDrug pipeline: At present, three double-antibody ADC pipelines, namely DM001, DM002 and DM004, have been introduced from Besetto, all of which are in preclinical stage. It is expected that IND will be launched in January 2024, and then clinical trials will be conducted simultaneously in overseas and China. Sidao Medicine owns the intellectual property rights and global rights of three pipelines.
DM001 is an anti-ADC drug targeting both Trop2 and EGFR. Pharmacokinetic analysis in mouse serum showed that the half-life of DM001 was similar to that of isotype control, about 30% of MMAE was released after 14 days in mice, and DM001 showed good anti-tumor activity in CDX mouse model of non-small cell lung cancer.
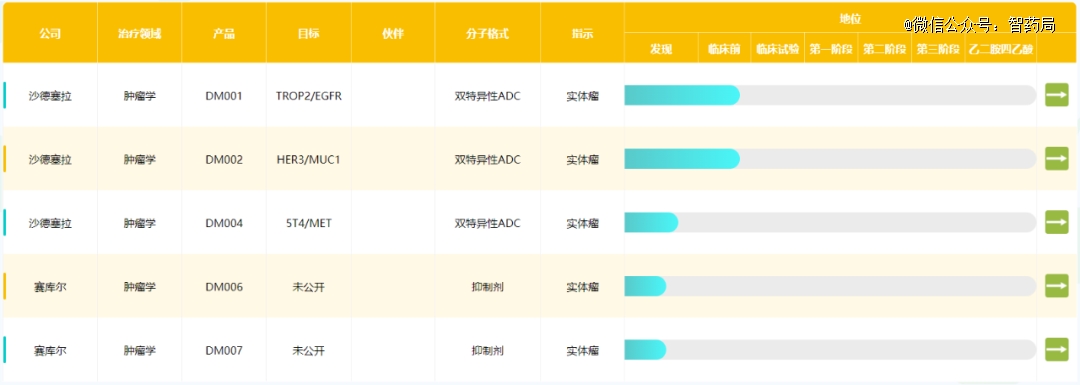
Figure: Sidao Medicine Clinical Pipeline