Research status and challenges of TCR-T cell therapy
0one
Foreword
Adoptive T cell transfer (ACT) is one of the most promising immunotherapy methods in tumor treatment.
At present, there are four mature ACT technologies, including autologous tumor infiltrating lymphocytes (TIL) therapy, antigen-specific endogenous T cell therapy (ETC), T cell receptor engineering T cell therapy (TCR-T) and chimeric antigen receptor T cell therapy (CAR-T).
TIL and ETC therapy rely on the isolation and expansion of T cells from tumor or peripheral blood respectively, while TCR-T and CAR-T therapy endow them with tumor antigen specificity by genetic modification of T lymphocytes.
At first, TCR-T cell therapy mainly targeted at common tumor-related peptide targets. With the recent development of technology, it has become possible to target new antigens from tumor somatic mutations, which represents a highly personalized treatment. TCR-T therapy has been used to test the clinical efficacy of solid tumors in many preclinical studies and clinical trials around the world.
However, the efficacy of TCR-T in the treatment of solid tumors still faces many challenges, including low TCR affinity, targeted toxicity and loss of target antigen that leads to tumor escape. At present, some new technologies and tools are being applied to TCR-T, which is helpful to improve the efficacy and safety of TCR-T therapy. TCR-T cell therapy is showing great potential for anti-tumor therapy.
02 Comparison between CAR-T and TCR-T
TCR molecule belongs to a superfamily of immunoglobulin, which consists of two covalently bound polymorphic subunits, each of which is antigen-specific, and they are related to at least four different types of signal transduction chains. In order to activate T cells, there must be interaction between TCR and MHC.
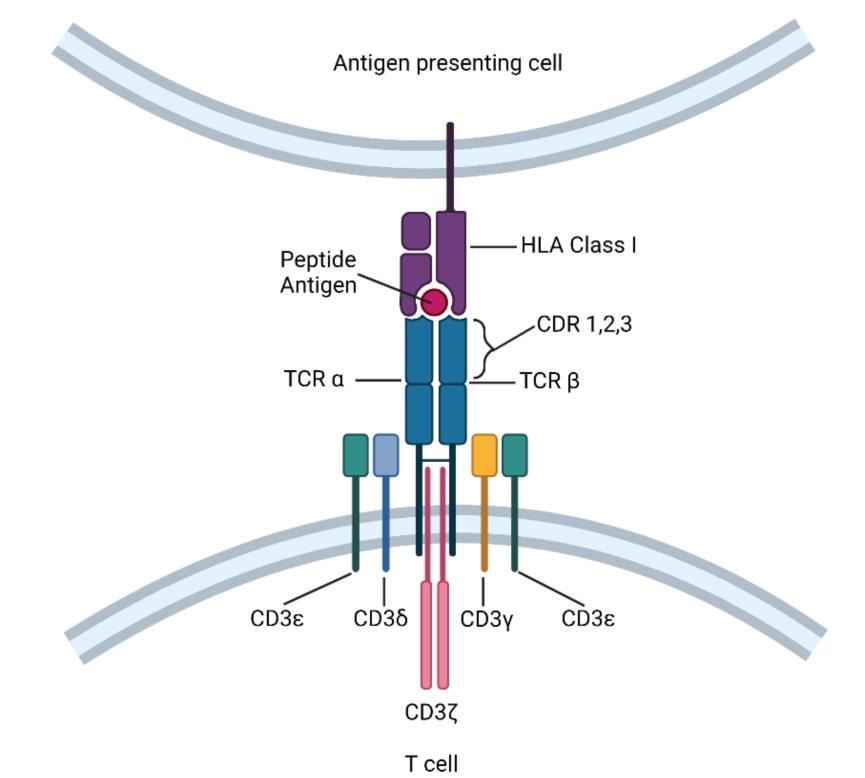
The interaction between TCRs and pMHC(peptide-MHC) determines the fate of immature thymocytes, which is very important for the survival of naive T cells. Therefore, TCR-T immunotherapy technology activates the host’s immune system through effective interaction with MHC, especially class II molecules. TCR-T cells can recognize tumor-specific antigens in cells, while CAR-T cells mainly recognize tumor-specific antigens on the surface. This makes TCR-T cells more effective in tumor treatment.
CAR contains single chain antibody targeting tumor antigen, transmembrane domain and intracellular activation domain of CD3ζ. In this way, the engineered CAR can recognize specific tumor-associated antigens, and CAR can bind untreated tumor surface antigens without MHC treatment.
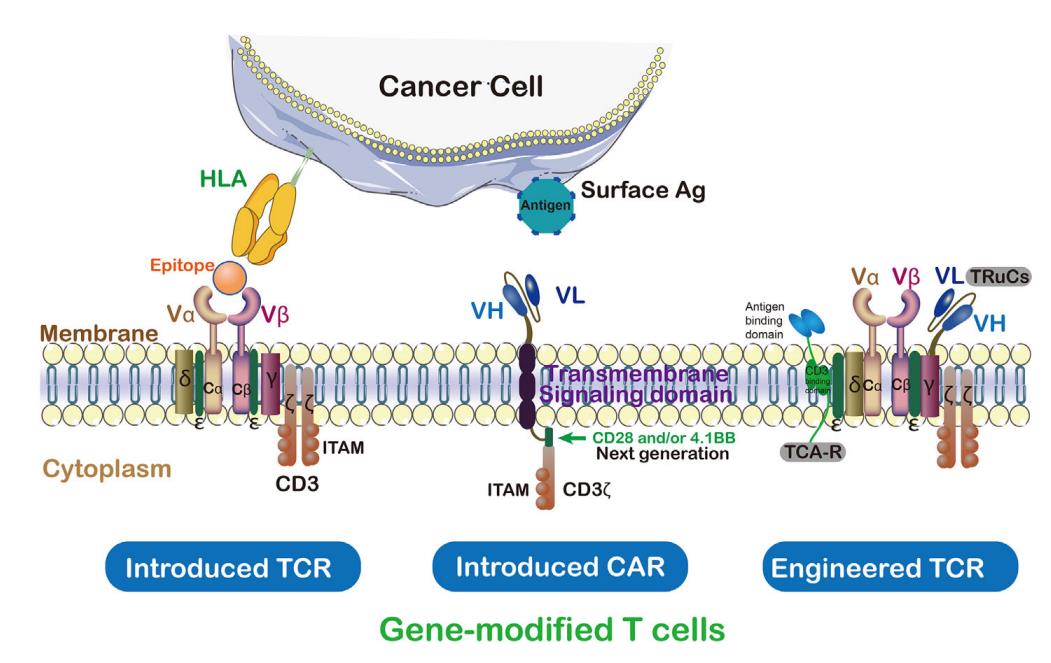
On the contrary, TCR is an α/β heterodimer that binds to MHC antigen complex. Compared with TCR, CARs has some disadvantages in identifying tumor antigens, such as extratumoral toxicity.
Compared with CARs, TCRs has some structural advantages in T cell-based therapy, such as more subunits (10: 1) in its receptor structure, more tyrosine-based activation motifs (ITAMs) in immune receptors (10: 3), less dependence on antigens (1: 100), and more costimulatory receptors (CD3, CD4, CD28, etc.). TCR with a low MHC affinity range (104-106M-1) can effectively activate T cells, whereas CARs needs a higher affinity range (106-109M-1).
Therefore, CAR-mediated cytotoxicity depends on higher density of cell surface antigens. In addition, T-cell/antigen interaction IS initiated in the structure of immune synapse (IS), in which TCR presents an annular region with peripheral LFA-1 adhesion, while CAR presents a diffuse LFA-1 distribution without annular region. Therefore, TCR-IS is slower but lasts longer than CAR-IS. At the same time, CAR-T cells show faster killing function and migrate to the next tumor target (serial killing), which is in sharp contrast to TCR-T cells’ prolonged signal transduction and killing time.
03
Clinical status of TCR-T cell therapy
As of August 9, 2021, there are 175 studies using TCR-T therapy in ClinicalTrials, 71 of which are specific TCR for specific TAA or new antigen, and 32 studies have been completed.
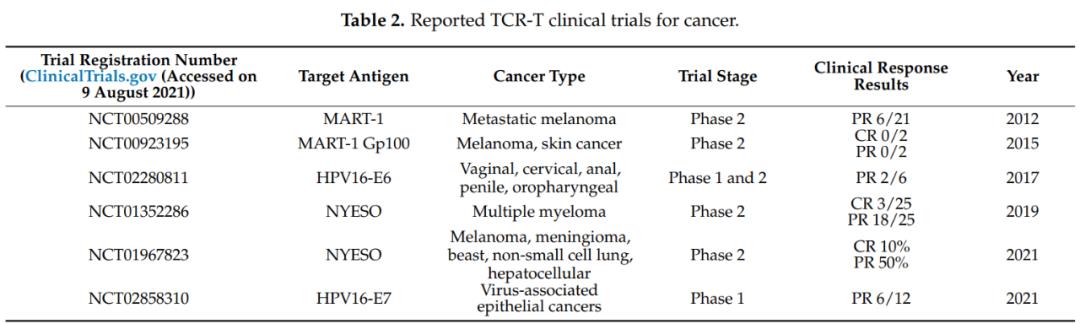
NY-ESO-1 is the most common targeted antigen, which is expressed in many cancers, including myeloma and melanoma. Other tumor testis-associated antigens, such as PRAME and MAGE proteins, melanoma differentiation antigens MART-1 and gp100, and recent cancer drivers, such as WT1, KRAS and TP53, are also popular TCR-T targets.
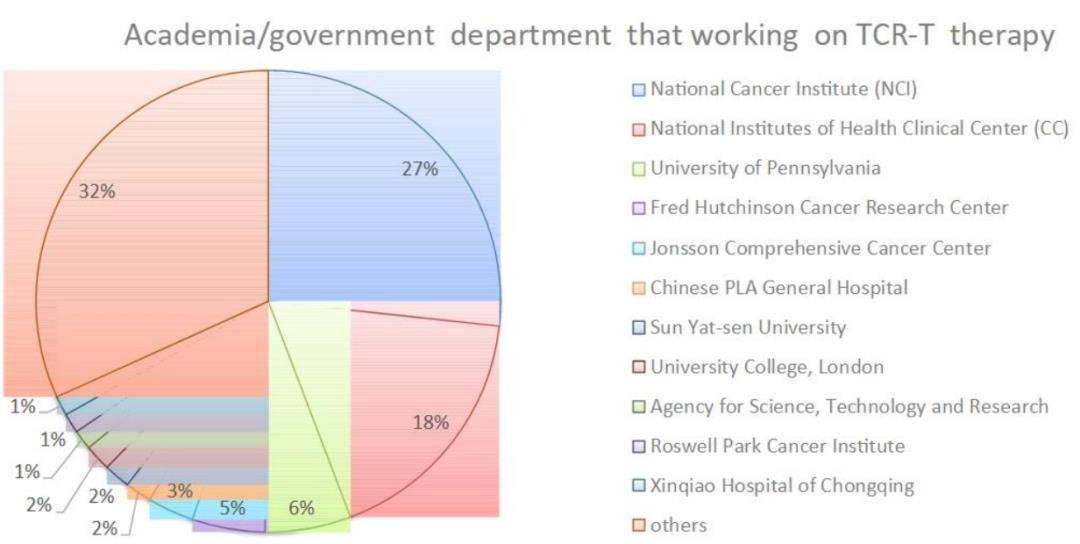
A total of 83 sponsors/collaborators initiated or participated in the research of TCR-T cell therapy, including the National Institutes of Health (NIH), government organizations, industries and universities/academic institutions. At present, the National Cancer Institute (NCI) has supported 53 TCR-T projects, accounting for 20% of all ongoing projects.
Among the 29 pharmaceutical companies developing TCR-T therapy, GlaxoSmithKline and Adaptimunime initiated the most clinical trials, with 11 and 7 respectively.
Recently, a phase 1 clinical trial (NCT02858310) of TCR-T cells targeting human papillomavirus (HPV)-16 E7 protein in the treatment of metastatic human papillomavirus-related epithelial cancer was reported. In this study, 6 of the 12 patients who received treatment showed objective clinical response and observed steady tumor regression.
This is a landmark clinical trial of TCR-T cell therapy, which proves that targeted virus antigen has good clinical effect on patients with virus-related cancer. Other viral antigens explored as TCR targets include HPV-E6 protein, antigens from Epstein-Barr virus (EBV) and human endogenous retrovirus (HERV) targets, such as Herv-E.
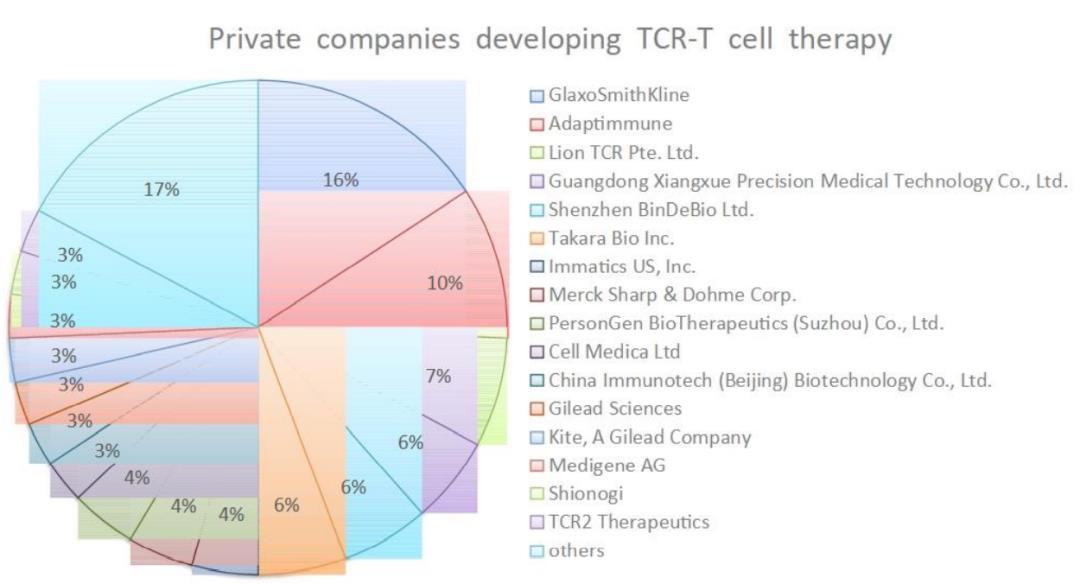
MART-1 and NY-ESO-1 TCR-T therapy targeting TAAs also showed clinical efficacy in advanced melanoma, myeloma and non-small cell lung cancer. The total effective rate (ORR) of TCR-T clinical trial is between 0 and 60%. It is worth noting that most of these TCR-T clinical trials only enrolled a small number of patients (2 to 25), so ORR may not be statistically accurate. Therefore, larger-scale phase II and III clinical trials are needed to confirm the actual clinical efficacy of these TCR-T therapies.
04
Challenges and potential solutions of TCR-T cell therapy
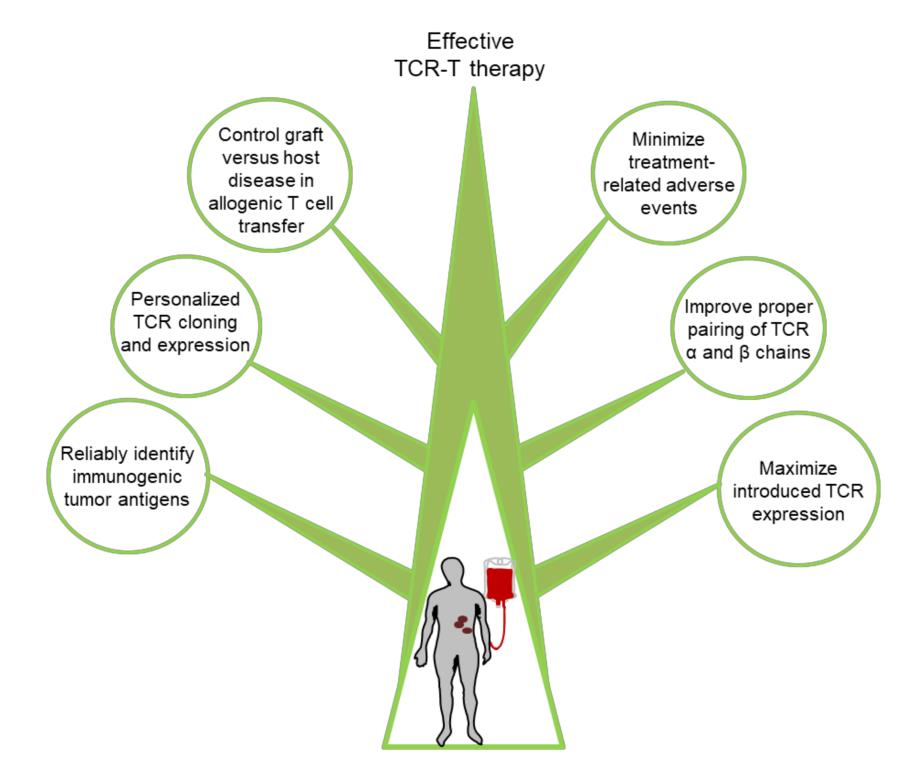
Although immunotherapy based on TCR-T cells has shown certain clinical efficacy in most patients receiving treatment, it still faces many challenges in many fields. These challenges include:
(1) Immunotoxicity caused by targeting normal tissues;
(2) The expression of TCR in engineered T cells is insufficient or transient;
(3)T cell exhaustion and dysfunction;
(4) tumor immune escape,
(5) Most cancer patients lack effective tumor-specific antigens as targets.
Overcoming these challenges will be the key to greater clinical success in the future.
Discovery of new targets
At present, the target of peptide antigen for effective and safe immunotherapy of TCR-T is very limited. At present, most of the targets used are TAA. Although it is up-regulated in tumor tissues, it still maintains a low level of expression in normal tissues, which may lead to autoimmune toxicity. Therefore, the new antigen seems to be the safest target for TCR-T cancer treatment.
However, the main challenges in developing new antigens in TCR-T clinic include:
(1) The mutation of new antigen formation is individualized to a great extent, and there are differences among cancer patients, so it is difficult to develop widely used immunotherapy products;
(2) The expression of new antigens in tumor tissues is often heterogeneous.
Nevertheless, recent reports have highlighted the emergence of new immunogenic antigens widely shared by tumor cells, including mutated KRAS and TP53. Many other studies have also demonstrated the immunogenicity of shared new antigens that can be used to generate potential therapeutic tumor-specific TCR.
With the development of next generation sequencing technology, especially single cell DNA sequencing, transcriptome sequencing and mature in vitro verification methods, TCR-T immunotherapy targeting personalized new antigens may become a popular cancer treatment method in the next few years. In addition, the emerging TAA class, such as carcinoembryonic antigen, may also constitute a feasible target for the future development of TCR-T.
Maximize therapeutic TCR expression
The correct pairing of transgenic α and β chains is one of the main challenges that hinder the development of TCR-T cells. Since each transduced T cell includes two endogenous TCR chains and two transformed TCR chains, heterodimers with unknown specificity can lead to potential autoimmune consequences. Another related problem is that improper α/β chain TCR pairing will compete for CD3 complex, thus reducing the surface expression and signal transduction of therapeutic TCR.
There are several ways to properly pair the transduced TCR chains, including:
(1) The constant region of partially murine TCR;
(2) adding cysteine residues to promote the introduction of disulfide bonds in TCR chains;
(3) changing the secondary structure of the constant region of endogenous TCR;
(4) adding a signal domain to the intracellular part transduced with TCR;
(5) Introducing TCR-α/β chain into substitute effector cells or constructing single-stranded TCR.
Methods for enhancing therapeutic TCR expression include:
(1) Codon optimization of TCR-α and TCR-β chain transgenes, and
(2) Changing the configuration of TCR-α/TCR-β vector to optimize expression.
Reduce adverse events
Usually, targeting non-tumor toxicity is the main key obstacle of TAA, and this risk urges researchers to study common new antigens more carefully. At present, several oncogene hot spot mutations are being studied as potential TCR targets, such as phosphoinositide -3- kinase (PI3K), KRAS and TP53.
In addition, genetically engineered TCR-T cells with suicide genes are an important safety measure. Obviously, it is very important to develop reliable, identifiable, personalized, highly specific and immunogenic tumor antigen targets to reduce adverse events related to TCR-T cell therapy.
Graft-versus-host disease of allogeneic T cells
Using allogeneic T cells is a very promising scheme, which can overcome manufacturing problems, patient-related immune cell defects and treatment delays. In order to use allogeneic T cells, it is necessary to control graft-versus-host disease caused by transduced allogeneic reactive lymphocytes and rejection of engineered lymphocytes by host immune system.
The deletion of endogenous TCR gene, HLA-I locus or CD52 molecule is one of the strategies to avoid the failure of TCR-T transplantation, which can be achieved by many methods, such as gene editing or using siRNA. In addition, pluripotent stem cell technology is also considered as a potential solution.
Conclusion
TCR-T therapy is a promising immunotherapy for cancer, which has incomparable advantages over other T-cell adoptive therapies. However, there are still several key challenges to improve the anti-tumor efficacy of TCR-T immunotherapy, including how to safely increase the affinity of therapeutic TCR, how to identify the common tumor-specific antigen and TCR in the patient population, and how to regulate the expression of TCR and achieve the best function. The solution of these problems will help to give full play to the potential of TCR-T cell therapy and bring hope to cancer patients to relieve their pain.
References:
1.Evolution of CD8+ T Cell Receptor(TCR) Engineered Therapies for the Treatment of Cancer. Cells. 2021 Sep; 10(9): 2379.
Shengming
The content of the article is for reference only and does not constitute investment advice. Investors operate accordingly, at their own risk, remain neutral about the statements and opinions in the text, and do not provide any express or implied guarantee for the accuracy, reliability or completeness of the contents. Readers are requested for reference only, and please take full responsibility; All kinds of articles published in WeChat official account focus on sharing. If there is any infringement, please contact us and we will deal with it.
Small medicine says medicine | source
Small medicine says medicine | author
Dara | compilation